
Get the free fda form 3640
Show details
Form Approved OMB No. 0910-0025 Expiration Date January 31 2017 FORM FDA 3640 3/14 Reporting Guide for Laser Light Shows and Displays Public reporting burden for this collection of information is estimated to average 24 hours per response including the time for reviewing instructions searching existing data sources gathering and maintaining the data needed and completing and reviewing the collection of information. Send comments regarding this bu...
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign fda form 3640
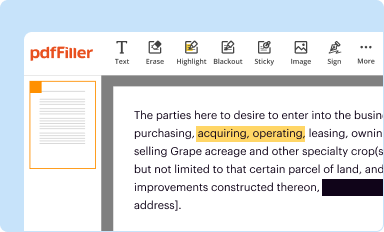
Edit your fda form 3640 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
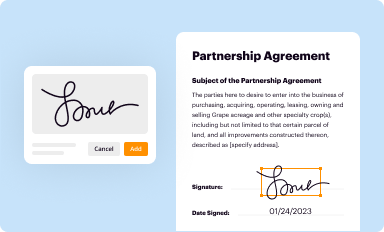
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fda form 3640 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing fda form 3640 online
Follow the guidelines below to use a professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit fda form 3640. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out fda form 3640
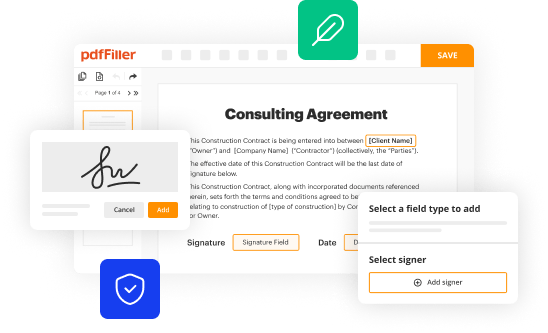
How to fill out FDA Form 3640:
01
Start by reviewing the instructions for completing FDA Form 3640.
02
Gather all the necessary information and documentation required for the form.
03
Begin filling out the form by providing your personal information such as name, address, and contact information.
04
Follow the instructions on the form to provide details about the product or activity that the form pertains to.
05
Be thorough and accurate when providing information about the product, including its intended use and any applicable regulations or guidelines.
06
Include any required supporting documentation or attachments as specified in the instructions.
07
Double-check all the information you have entered to ensure its accuracy and completeness.
08
Sign and date the form as required.
09
Submit the completed FDA Form 3640 according to the instructions provided.
Who needs FDA Form 3640:
01
Manufacturers or distributors of certain products regulated by the Food and Drug Administration (FDA) may need to fill out FDA Form 3640.
02
This form is typically required for activities such as exporting food, drugs, cosmetics, medical devices, or radiation-emitting electronic products.
03
Individuals or businesses involved in importing or exporting these regulated products may also be required to complete FDA Form 3640.
04
It is essential to consult the specific FDA regulations or guidance documents pertaining to your product or activity to determine if you need to fill out this form.
05
The purpose of this form is to provide the FDA with important information about the products or activities being conducted to ensure compliance with applicable laws and regulations.
Fill
form
: Try Risk Free
People Also Ask about
What are the regulations for laser projectors?
Under U.S. FDA regulations for laser light shows, any laser beam above 1 mW cannot be lower than 3 m (10 ft) from the floor or other surface on which a person could reasonably be expected to stand. If your ceiling is below 3 meters, you could not legally use the laser projector.
Do I need a laser variance?
Any laser demonstrations, displays or shows that use lasers above 5 milliwatts must have a “variance” from FDA. This document gives the variance holder permission to “vary” from the 5 milliwatt limit, by using more power.
What are the FDA regulations for laser projectors?
Industry Guidance Laser products promoted for demonstration purposes are limited to hazard Class IIIa by FDA regulation 21 CFR 1040.11(c). This means that projectors are limited to 5 milliwatts output power in the visible wavelength range from 400 to 710 nanometers.
Are there any risks with a laser is laser harmful to humans?
Lasers can be hazardous if appropriate controls and safe systems of work are not used. Laser beams may cause damage to eyes or skin. The risk of eye injury from laser light and heat is particularly of concern as eyes focus and intensify light entering them.
Are lasers regulated by FDA?
The FDA has the authority to regulate all kinds of lasers. Under the Medical Device Amendments to the Federal Food, Drug, and Cosmetic Act, the agency regulates lasers used in medicine.
Why does FDA regulate lasers?
The FDA may inspect manufacturers of laser products and require the recall of products that don't comply with federal standards or that have radiation safety defects. The agency also may test laser products and inspect displays of laser light shows to ensure the public is protected.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send fda form 3640 for eSignature?
When your fda form 3640 is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
How can I edit fda form 3640 on a smartphone?
The pdfFiller mobile applications for iOS and Android are the easiest way to edit documents on the go. You may get them from the Apple Store and Google Play. More info about the applications here. Install and log in to edit fda form 3640.
How do I fill out fda form 3640 using my mobile device?
The pdfFiller mobile app makes it simple to design and fill out legal paperwork. Complete and sign fda form 3640 and other papers using the app. Visit pdfFiller's website to learn more about the PDF editor's features.
What is fda form 3640?
FDA Form 3640 is a form used by manufacturers, packers, and distributors to report adverse events associated with dietary supplements.
Who is required to file fda form 3640?
Manufacturers, packers, and distributors of dietary supplements are required to file FDA Form 3640.
How to fill out fda form 3640?
To fill out FDA Form 3640, you need to provide information about the dietary supplement, the adverse event, and contact information. It is important to include all relevant details.
What is the purpose of fda form 3640?
The purpose of FDA Form 3640 is to enable the FDA to monitor and evaluate the safety of dietary supplements by collecting information about adverse events.
What information must be reported on fda form 3640?
FDA Form 3640 requires reporting of information such as the name and contact information of the reporter, the name of the dietary supplement, details of the adverse event, and any medical treatments sought.
Fill out your fda form 3640 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Fda Form 3640 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.



























